Research
Hippo Signaling
The Hippo signaling pathway is a relative newcomer in the field of signal transduction, but it has emerged as a major regulator of organ growth. We are interested in how downstream signals are interpreted in this pathway by the transcriptional coactivator Yorkie (Yki), a fly homolog of human YAP/TAZ proteins. Novel regulators of this pathway identified in our laboratory in AP-MS experiments have been biologically validated in collaborative work with the Moberg and Harvey labs (Gilbert et al., 2011; Dent et al., 2015; Zhang et al., 2015; Poon et al., 2018). Our current work is focused on the protein interaction network of Yki, with the goal of identifying new components and mechanisms. One of the novel members of this network that we identified is a transcriptional regulator Bonus (Bon). In a surprising twist, we have found that Bon and Yki are likely involved in cell fate specification rather than growth, thus increasing the range of functions under Yki regulation (Zhao et al., 2023). We are currently continuing our studies of the novel Yki interactors.
Erk Signaling
The extracellular signal-regulated kinase (ERK) is a member of a conserved RAF-MEK-ERK signaling module that is a focal point of regulation by multiple upstream signals. ERK plays a critical role in a wide range of developmental events, such as cell proliferation, differentiation, and apoptosis. An important and unresolved question is how ERK exerts its activity over a large number of substrate proteins that it targets for phosphorylation. One of the key sensors of ERK activity is Capicua (Cic), a transcriptional repressor that is inactivated by ERK signaling in flies and mammals. Recent research has established human CIC as a tumor suppressor that is lost or dysregulated in many cancers. We discovered that Cic binds to activated phosphorylated ERK with higher affinity, compared to inactive ERK, and that this preferential binding is important for signal propagation in the ERK pathway (Paul et al., 2020). Current efforts are directed at understanding the molecular and developmental consequences of Cic phosphorylation by ERK.
Minibrain functions
The kinase Minibrain (Mnb) is homologous to the human DYRK1A protein, which is implicated in Down syndrome, microcephaly, and other human diseases. We initially characterized Mnb as a growth regulator and a new component of the Hippo pathway (Degoutin et al., 2013). In subsequent work, we found that Mnb downregulates Cic function in parallel to ERK, which expanded our understanding of Mnb's role in controlling organ growth and tissue patterning (Yang et al., 2016). More recently, we identified a new signaling axis involving Mnb and the trafficking regulators Reps, Rlip and Rala (Brown et al., 2024). We are hoping that through the studies of Mnb functions in flies we would gain insight on how DYRK1A regulates the development of the brain and other organs in mammals.
Developmental proteomics
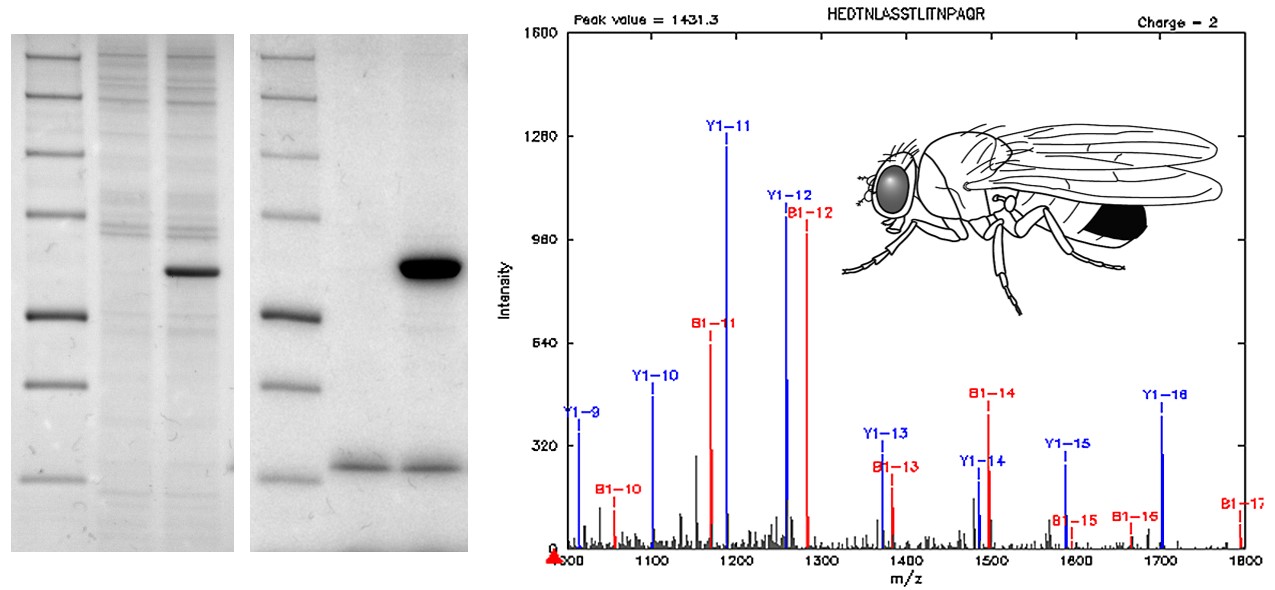
Developmental signaling pathways rely on multiple protein-protein interactions that are involved in signal transmission and interpretation. To discover novel components of signaling pathways, we use affinity purification-mass spectrometry (AP-MS). This method involves expression of a tagged protein of interest in cell culture or in vivo, followed by purification of protein complexes and analysis of interactions by mass spectrometry (Veraksa, 2010; Veraksa, 2013; Yang et al., 2017; Yang and Veraksa, 2017). After this initial identification, which is analogous to running a genetic screen, interactions are validated in flies using genetic, imaging, and biochemical methods. These approaches have led to discoveries of novel signaling components in the Notch pathway, receptor tyrosine kinase/ERK, and Hippo/Yorkie signaling pathways. Our laboratory also serves the scientific community by distributing DNA vectors for use in protein tagging and purification, as well as by sharing proteomics expertise.
Beta-arrestin Kurtz
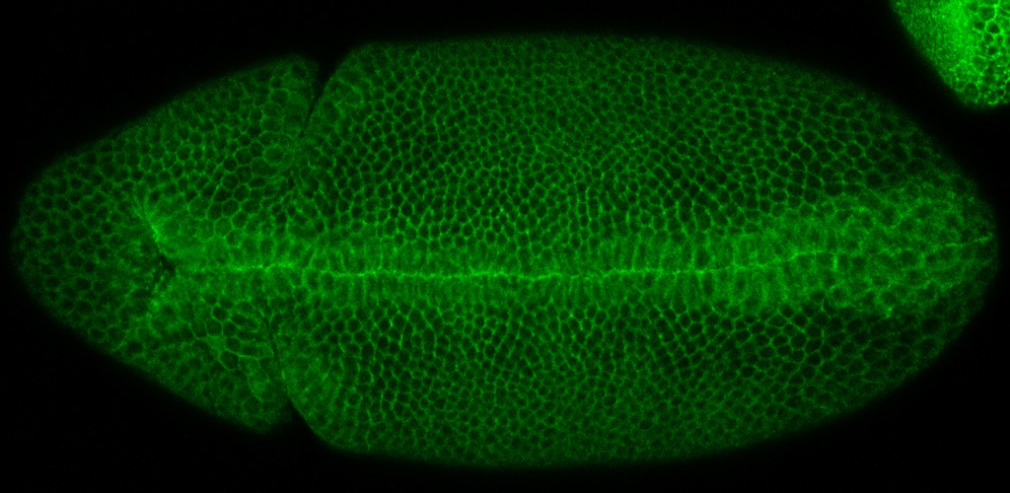
Our laboratory has made contributions to the analysis of signaling functions of the Drosophila β-arrestin Kurtz (Krz). Krz and its mammalian homologs desensitize and also signal downstream of G protein coupled receptors (GPCRs). Work in our lab has revealed the role of Krz in regulating the receptor tyrosine kinase Torso and Toll/Dorsal (NF-κB) signaling pathways in Drosophila embryogenesis. We have found that Krz inhibits ERK signaling by a previously unknown sequestration mechanism (Tipping et al., 2010). We have also shown that Krz plays an important role in controlling immune homeostasis in Drosophila larvae by limiting Toll pathway activity, which occurs via an association of Krz with a SUMO protease Ulp1 (Anjum et al., 2013). We also identified residues in Krz that are critical for its role in limiting the activity of the GPCR Fog-Mist signaling pathway during epithelial morphogenesis (Chai et al., 2019).